Freshly ground silica is more biologically reactive than aged silica. Fracture-generated silicon-based radicals may play a significant role in the pathogenesis of lung disease. Research has shown that freshly fractured and geologically old (aged) quartz behave differently due to the biological activity of these materials. Studies show that quartz found in the bentonite was significantly less toxic than quartz, indicating that surface reactivity of quartz found in bentonite is considerably less reactive than that of freshly fractured quartz. The research studies show that the toxicological properties of quartz particles can vary significantly dependent on their surface characteristics. Toxicity can range from modest to a severe and persistent inflammatory state caused by ground quartz with fractured surfaces. This research was the basis for the exemption for bentonite from the OSHA Rule on Occupational Exposure to Respirable Crystalline Silica.

Bentonites are non-consolidated sedimentary rocks and belong to the silica clay minerals which are important for industry. Bentonite is an absorbent swelling clay consisting mostly of montmorillonite. It usually forms from weathering of volcanic ash in seawater,1,2 which converts the volcanic glass present in the ash to clay minerals. The term bentonite does not describe any mineral phase, but a mineral conglomeration of different compositions in which mineral phase montmorillonite occurs in different proportions having different functional importance. There are two main types of bentonite, which are named after the respective dominant cation,3 sodium and calcium bentonite. Sodium bentonite is the more valuable, but calcium bentonite is more common.4
As a swelling clay, bentonite can absorb large quantities of water, which increases its volume by up to a factor of eight. The swelling property is used to enhance drilling mud and groundwater sealants. Bentonite is used in a variety of pet care items such as cat litter to absorb pet waste. It is also used to absorb oils and grease.

The montmorillonite making up bentonite is an aluminum phyllosilicate mineral, which takes the form of microscopic platy grains. These give the clay a large total surface area, making bentonite a valuable adsorbent. The plates also adhere to each other when wet. This gives the clay a cohesiveness that makes it useful as a binder and as an additive to improve the plasticity of kaolinite clay.
Bentonite itself is not classified as a carcinogen by any regulatory or advisory body, but some bentonite may contain variable amounts of respirable crystalline silica (quartz), a recognized human carcinogen. Inhaled quartz has long been known to cause health problems. Research on exposure and silica species characterization led to issuance of a Safe Use Determination in 1999 by the California Office of Health Hazard Assessment for respirable crystalline silica in mineral-based (bentonite) cat litter.5 Based on epidemiological and toxicological research, the International Agency for Research on Cancer (IARC) stated in their 1997 monograph on crystalline silica that there was “sufficient evidence in humans for the carcinogenicity of inhaled crystalline silica in the form of quartz or cristobalite from occupational sources.” This finding caused IARC to rank quartz as a known carcinogen (Class 1), which is the highest cancer ranking.6
Crystalline silica in the form of quartz is the second most common mineral in the crust of the earth. People are in intimate contact with quartz every day. This type of exposure is a benign one. However, it is recognized that there is a connection between silicosis, a disease resulting from progressive fibrosis of the lung tissue, and the inhalation of quartz in certain industrial circumstances. IARC noted that carcinogenicity may be dependent on the inherent characteristics of the crystalline silica or on external factors affecting its biological activity or distribution of its polymorphs. In other words, IARC found that inhaled quartz causes cancer but only in some circumstances.
It is known that the reactivity of solid materials is usually dependent on their surface properties. Most quartz particles found in the environment are derived from natural weathering of quartz bearing rock. The weathering process results in quartz particles having geologically old surfaces that are in chemical equilibrium with their surroundings with little chemical reactivity. The surfaces of these weathered particles are not composed of SiO2 but have a microscopically thin layer of a variety of materials such as water molecules, carbon compounds, aluminum ions, and magnesium ions which attached during their formation. Although only a few microns in thickness, this thin layer coats the quartz effectively creating a barrier to the silica’s surrounding environment.
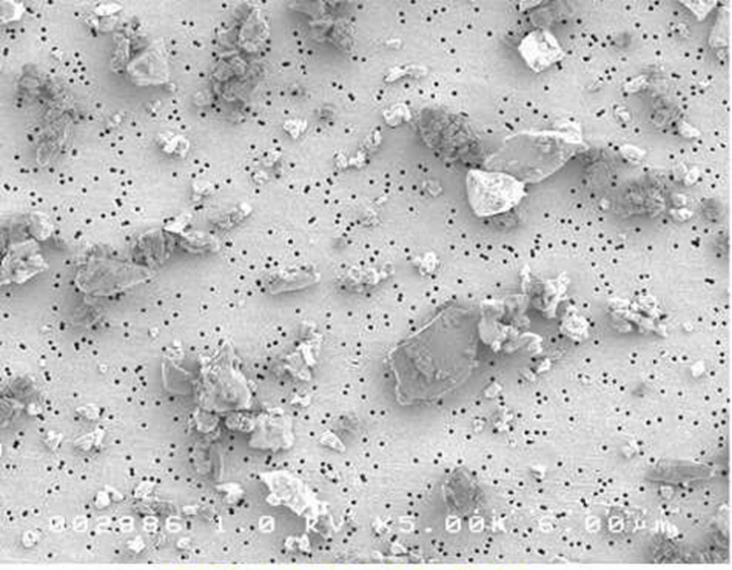
Some industrial processes can fracture the quartz grains into smaller particles. When these particles are fractured the fresh surfaces become highly chemically reactive. When the silica is freshly cleaved, the chemical bonds are broken, and the new surface atoms (free radicals) are seeking to chemically bond to other atoms. These exposed free radicals can be very biologically damaging in lungs. It is theorized that these piezoelectric characteristics may play a role in the pathophysiology of silica-related illness by the generation of oxygen free radicals produced on the cleaved surfaces of silica molecules and as a result of silica-damaged alveolar macrophages.7,8 Silanol (SiOH) groups present on the surface of silica particles are capable of forming hydrogen bonds with oxygen and nitrogen groups found in biologic cell membranes, which then may lead to a loss of membrane structure, lysosomal leakage, and tissue damage. These processes may all contribute to the development of lung scarring.7
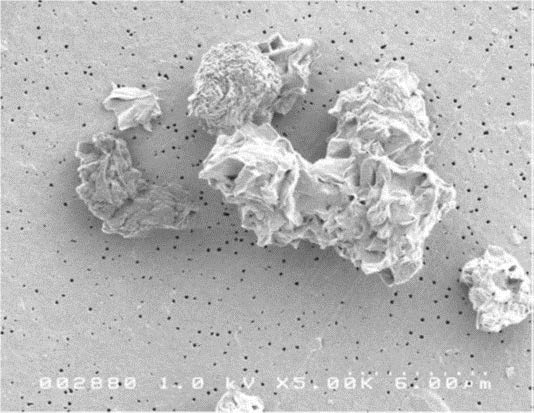
Very small particles, less than 3 microns in size, may penetrate sufficiently deep enough into the lung reaching the alveoli region. This is the where the gas exchange takes place with the blood cells. Specialized cells in the lung called alveoli macrophages engulf the particles that reach the alveoli through the process of phagocytosis. The macrophages then migrate out of the alveoli and into the fine branches of the bronchial tree where they are removed from the lung. If the silica particles are highly reactive, they can rapidly kill the macrophages, causing them to rupture and release the particle and a variety of reactive cellular chemicals. These chemicals will cause inflammation eventually leading to fibrosis. According to IARC, cancer may be caused by this inflammatory process. However, if the quartz particles have hydroxylated surfaces, damage to the macrophages is will be significantly reduced, although some damage will occur. This will significantly reduce the amount of inflammation in the lung as the macrophages will be able to effectively remove a large amount of the particles before they are killed and subsequently rupture. Quartz particles having occluded surfaces produce minimal damage to the macrophages. This is because the thin barrier layer formed on the surface of the silica particle effectively prevents chemical interaction with the macrophage.
Research data indicate that freshly fractured silica exhibits surface characteristics and biologic reactivity distinct from aged silica, and on this basis, it was proposed that these surface features may lead to enhanced manifestations of lung injury.9 Grinding of silica produces ∼1018 Si and Si-O (silicon-based) radicals per gram of dust on the particulate surface. The number of free radicals declines over time as they begin to age due to hydration of the quartz surface. The silicon-based radicals react with aqueous media to produce OH radicals. The concentration of silicon-based radicals in silica decreases with aging in air and exhibits a half-life of ∼30 h, whereas its ability to generate OH radicals in aqueous solution decreases with a half-life of ∼20 h. However, on storage in aqueous media, the concentration of silicon-based radicals and the dust’s ability to generate OH radicals decrease significantly within a few minutes. A fully hydrated surface exhibits little if any chemical reactivity.
Freshly ground silica is also more biologically reactive than aged silica. Because acute silicosis is frequently associated with occupations in which freshly fractured crystalline silica of respirable size is generated, research9 suggests that fracture-generated silicon-based radicals may play a significant role in the pathogenesis of this disease.
Research has shown that freshly fractured and geologically old (aged) quartz behave differently due to the biological activity of these materials.10 A study was performed to characterize the differences in biological activity between different quartz species of similar particle size.11 The quartz investigated in this study was derived from a sodium bentonite deposit formed over 100 million years ago. The reference quartz in the positive control was freshly crushed quartz that has been accepted as a biologically active standard in other toxicological studies.12,13 The physicochemical properties of the quartz removed from the bentonite clay and the reference quartz sample reveal that these materials were substantially different. The reference quartz had a much more electrically reactive surface than did the quartz from the bentonite clay. The mineralogical composition shows the reference quartz to be nearly pure silicon dioxide while the quartz removed from the bentonite contains 30% montmorillonite clay as an inseparable coating on the surface of the quartz particles. Additionally, the morphological characteristics of particles of these materials are also quite different. These differences provide some physicochemical basis for the differences observed in the overall toxicological results. Quartz found in the bentonite was significantly less toxic than quartz, indicating that surface reactivity of quartz found in bentonite is considerably less reactive than that of freshly fractured quartz.
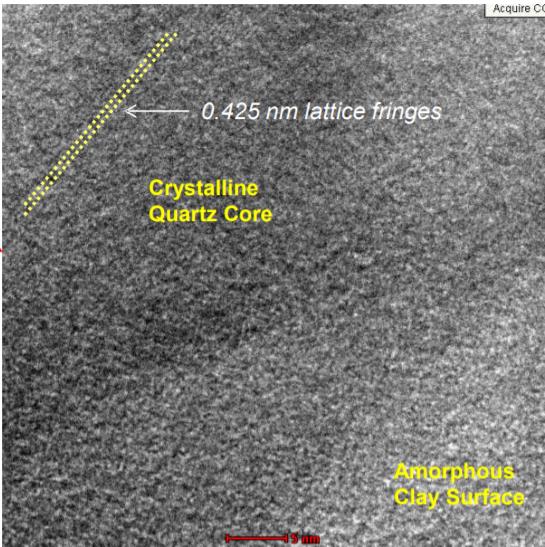
The research studies show that the toxicological properties of quartz particles can vary significantly dependent on their surface characteristics. Toxicity can range from modest to a severe and persistent inflammatory state caused by ground quartz with fractured surfaces. This research was the basis for the exemption for bentonite from the OSHA Rule on Occupational Exposure to Respirable Crystalline Silica.14

Nesse, William D. (2000). Introduction to mineralogy. New York: Oxford University Press. p. 257. ISBN 9780195106916.
Sutherland, Wayne M. (September 2014). “Wyoming Bentonite” (PDF). Wyoming State Geological Survey. Retrieved 12 January 2021
T. Brown et al. 2020. World Mineral Production 2014–18. British Geological Survey, Nottingham, England.
Anderson, Duwayne M.; Hoekstra, Pieter (1965). “Migration of Interlamellar Water During Freezing and Thawing of Wyoming Bentonite,” Soil Science Society of America Journal. 29 (5): 498.
Figure 2. Bentonite clay is “clumping” cat litter. This type of clay can swell up to 15 times its original volume when wet Issuance of a Safe Use Determination for Crystalline Silica in Sorptive Mineral-based Pet Litter, Jun 4, 1999, California OEHHA, proposition 65.
International Agency for Research on Cancer, 1997, IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to humans: Silica, Some Silicates, Coal Dust, and para-Aramid Fibrils, 68, 41-242.
Williamson BJ, Pastiroff S, Cressey G. Piezoelectric properties of quartz and cristobalite airborne particulates as a cause of adverse health effects. Atmos Environ 2001; 35:3539-42
Castranova V. Signalling pathways controlling the production of inflammatory mediators in response to crystalline silica exposure: role of reactive oxygen/ nitrogen species. Free Radic Biol Med 2004;37(7):916-25.
Val Vallyathan , Xianglin Shi , Nar S. Dalal , William Irr , and Vincent Castranova, Generation of Free Radicals from Freshly Fractured Silica Dust: Potential Role in Acute Silica-induced Lung Injury, PubMed: 2849348, January 11, 1988.
Richard K. Brown, William F. Moll, Sarkis G. Ampian, William J. Miles and S. Lee Coogan, The Problem with Quartz – When is it a Health Hazard, and Why? Sorptive Minerals Institute, Washington, D.C., December 2003.
Rats O. Creutzenberg, T. Hansen, H. Ernst, and H. Muhle, G. Oberdorster, R. Hamilton, Inhalation Toxicology, Toxicity of a Quartz with Occluded Surfaces in a 90-Day Intratracheal Instillation Study in Rats, Inhalation Toxicology, 2008.
Robock, K. 1973. Standard quartz DQ12<5μmfor experimental pneumoconiosis research projects in the Federal Republic of Germany, Ann. Occup. Hyg. 16:63–66.
Clouter, A., Brown, D., H¨ohr, D., Borm, P., and Donaldson, K. 2001. Inflammatory effects of respirable quartz collected in workplaces versus standard DQ12 quartz: Particle surface correlates. Toxicol. Sci. 63:90–98.
OSHA Rule on Occupational Exposure to Respirable Crystalline Silica, Occupational Exposure to Respirable Crystalline Silica; Final Rule Federal Register / Vol. 81 , No. 58 / Friday, March 25, 2016 / Rules and Regulations, https://www.osha.gov/laws-regs/federalregister/2016-03-25-1